Ampoules are widely used in the pharmaceutical industry to store injectable drugs, vaccines, and other sterile liquids. Their simple glass design provides excellent protection against contamination when manufactured correctly. However, even minor defects can compromise sterility, leading to product loss, safety risks, and regulatory non-compliance.
Because of these risks, ampoule integrity testing has become a standard part of pharmaceutical quality control. Industry-focused solution providers such as SEAL-CHECK specialize in leak detection technologies designed to identify even microscopic defects before products reach the market. Understanding the most common causes of ampoule leakage helps manufacturers apply the right preventive and testing strategies at each production stage.
Why ampoule leakage is a critical issue
Ampoules are often used for products that are administered directly into the human body. Any loss of sterility can have serious consequences, including reduced product efficacy or potential health risks. Since ampoules are single-use containers, even a minimal leak is enough to render the product unusable.
Regulatory bodies expect manufacturers to demonstrate control over packaging integrity, making leak detection not only a quality issue but also a compliance requirement.
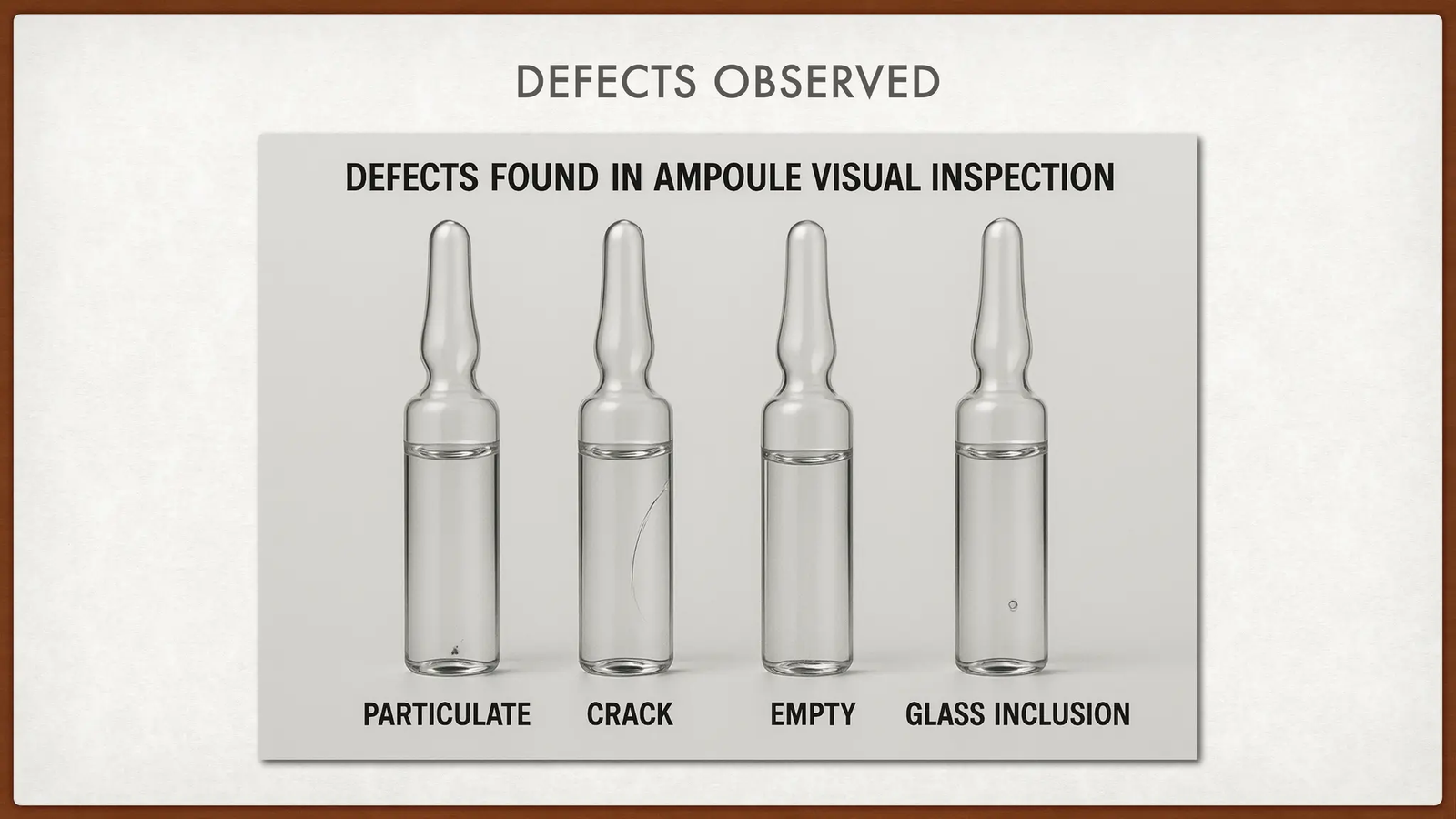
Manufacturing-related causes of ampoule leakage
One of the most frequent sources of leakage occurs during sealing. If flame-sealing parameters are not precisely controlled, microcracks or incomplete closures can form at the ampoule tip. Variations in glass composition or wall thickness further increase this risk.
Glass defects such as inclusions or internal stress may remain undetected during visual inspection but become critical during sterilization or transport. Filling inconsistencies also play a role, as incorrect fill volumes can create internal pressure that weakens the seal during closing.
External factors during handling and distribution
Even well-manufactured ampoules are vulnerable to external stress. Mechanical impacts during packaging and transportation can cause microfractures, particularly around the sealed neck. Temperature fluctuations during sterilization or cold-chain storage may also introduce thermal stress that compromises seal integrity over time.
These factors highlight why leak detection should not be limited to a single production step but considered throughout the entire product lifecycle.
How manufacturers detect and prevent ampoule leakage
Effective prevention relies on a combination of controlled processes and reliable testing. Pharmaceutical manufacturers typically implement several quality assurance measures to reduce leakage risk and detect defects early.
Key practices include:
- precise control of sealing parameters and equipment calibration
- inspection of incoming glass materials
- consistent filling volume management
- routine integrity testing to detect non-visible leaks
Among these measures, an ampoule leak test is essential for verifying seal integrity and identifying microleaks that visual inspection cannot detect. Such testing is commonly integrated into quality control workflows to ensure batch-to-batch consistency and regulatory compliance.
The role of modern leak detection technologies
As production volumes grow, manual inspection becomes increasingly limited. Automated leak detection systems provide higher accuracy, repeatability, and documentation. Companies like SEAL-CHECK focus on developing testing solutions that balance sensitivity with gentle handling, ensuring fragile ampoules are tested without introducing additional damage.
These technologies help manufacturers reduce waste, improve traceability, and maintain consistent quality standards across large-scale production.
Preventing leakage before it becomes a risk
Ampoule leakage is rarely caused by a single failure. Instead, it results from a combination of material, process, and handling factors. By understanding these risks and applying reliable detection methods early, manufacturers can significantly reduce product loss and safety concerns.
Integrating robust leak detection practices into quality control systems ensures that sterile products remain protected from production through final use, supporting both patient safety and regulatory confidence.